Overview | TME | FAP | Epigenetics | PDX
Fibroblast activation protein in prostate cancer progression
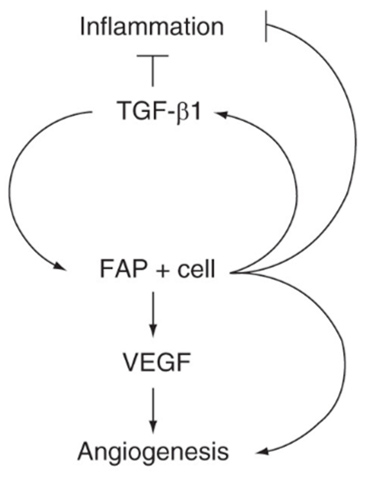
Fibroblast activation protein (FAP) in prostate cancer progression is a serine protease upregulated at sites of tissue remodeling and cancer that represents a promising therapeutic and molecular target in multiple tumor types. While its precise physiological role is still poorly understood, FAP has been implicated in multiple pro-tumorigenic pathways including proliferation, angiogenesis, invasion, metastasis, and immunosuppression. Increased expression is correlated with higher tumor grade and worse overall survival in several malignancies.
In prostate cancer, studies of FAP expression have been conflicting, such that its clinical potential is unclear. Though it has been associated with increased risk of biochemical recurrence and disease progression after prostatectomy.
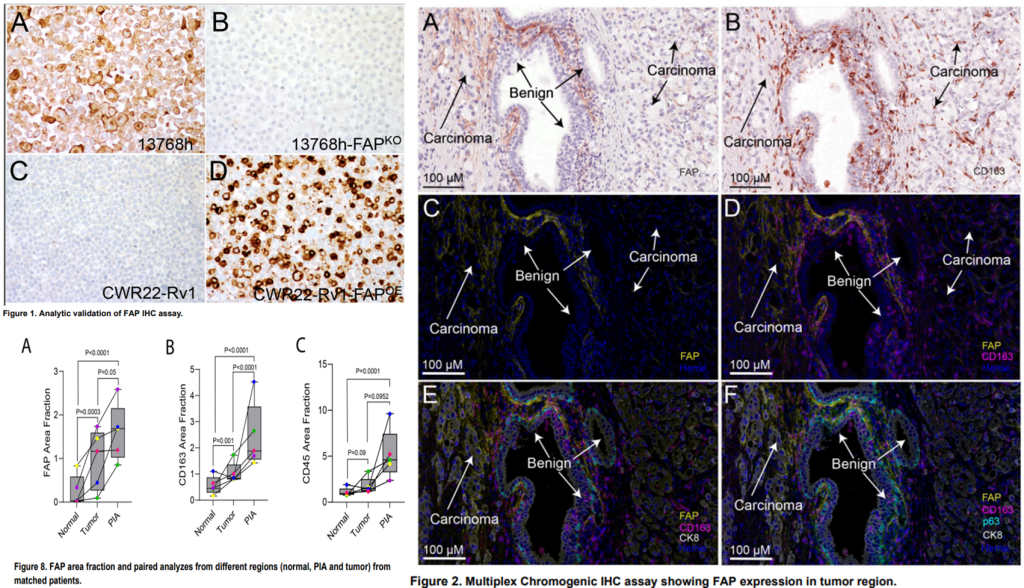
We have documented that while FAP is nearly absent in benign regions, it is consistently present in areas of proliferative inflammatory atrophy (PIA), a prostate cancer precursor legion. In prostate cancer, FAP was expressed in all cases, however, it was highly heterogeneous. High FAP levels were associated with increased pathological stage, cribriform morphology, and correlated with CD163+ M2 macrophage density.
Using transgenic syngeneic and patient-derived xenograft (PDX) models, we are investigating the mechanistic role of FAP in prostate cancer pathobiology and progression in the premalignant, primary, and metastatic setting. Additionally, we are evaluating the therapeutic activity of a series of FAP-targeted small molecule and peptide-based inhibitors as well as FAP-activated prodrugs/protoxins.