Overview | TME | FAP | Epigenetics | PDX
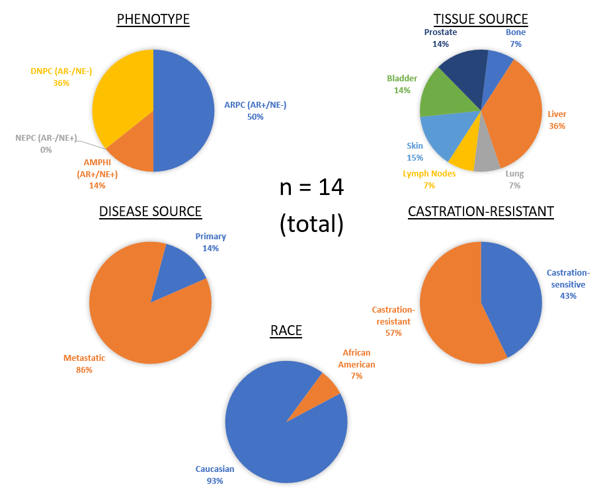
Developing novel patient-derived xenograft (PDX) models of advanced mCRPC
Patient-derived xenograft (PDX) models in which primary human tissue is serially propagated in immunocompromised murine hosts, are the gold-standard for the preclinical assessment of in vivo anti-cancer activity for new therapeutic agents and treatment strategies. This status is due to several advantages PDXs have over other current models for drug discovery –
- more accurately retain the molecular and pathological heterogeneity of patient tumors, potentially representing the full spectrum of disease observed clinically;
- offer a diverse platform to explore tumor biology, disease progression, and therapeutic response in a manner that reflects the clinical scenario;
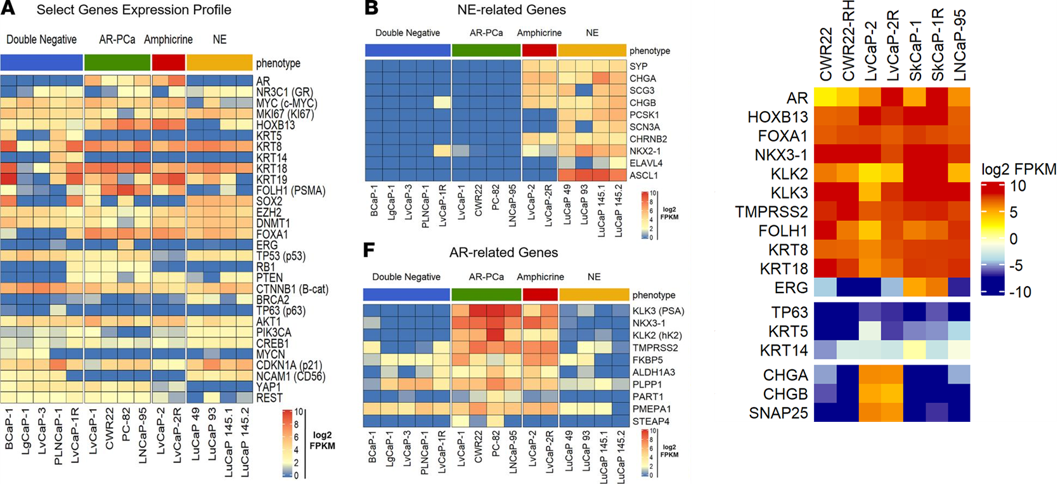
- treatment-induced resistance and emerging phenotypes such as neuroendocrine and double negative prostate (NEPC/DNPC) not robustly represented in the limited number of traditional cell line-derived models;
- opportunity to uncover subtype-specific treatment strategies, relating tumor biology to drug response and resistance;
- renewable resource enabling distribution for discovery and cross-validation of results, and 6. source of patient-derived organoids (PDOs) for high throughput screens and drug testing.
Our group has spent several years developing and characterizing new PDX models from mCRPC patients representing diverse molecular and pathological phenotypes with a broad range of responses and resistance mechanisms to standard-of-care and emerging therapies. We make these models are available to the broader research community in order to accelerate the development of effective approaches for the prevention, detection, and treatment of prostate cancer.